What is a clinical trial?
Clinical trials, also known as clinical research studies, are carefully designed investigations involving volunteer human participants to evaluate the safety, efficacy and outcomes of medical or surgical interventions. These interventions can include experimental drugs, devices, therapies, diagnostic tools, vaccines, procedures or lifestyle modifications such as diet or exercise.
Clinical trials are a fundamental component of medical research. They are typically funded or sponsored by government agencies (e.g., the National Institutes of Health), pharmaceutical companies, biotechnology firms, academic institutions or nonprofit organizations. Their primary goal is to generate scientific evidence that informs regulatory decisions, clinical practice and future research.
Why clinical trials are important
Clinical trials are critical for advancing medical science and public health. They help determine the following:
- Whether a new drug, device or treatment is safe and works as intended.
- How a treatment compares to standard therapies.
- What side effects may occur and how to manage them.
- Proper dosage levels and administration frequency.
- Drug interactions with food, other medications or health conditions.
By rigorously evaluating new interventions, clinical trials provide the data needed to balance benefits against risks. This evidence helps regulatory bodies assess new therapies and allows healthcare providers to make informed treatment decisions.
Regulation of clinical trials
Clinical trials are tightly regulated in the U.S to protect participants and ensure data integrity. In U.S.-based research, regulatory bodies perform different functions:
- Food and Drug Administration (FDA): The FDA evaluates the safety and effectiveness of drugs, biologics and devices.
- National Institutes of Health (NIH). The NIH is the world's largest public funder of biomedical research. It also sets guidelines for research conduct, data and safety monitoring, and other practices.
- Institutional Review Boards (IRBs). Typically based in the host institution conducting the trial, IRBs are administrative bodies that review and approve clinical trial protocols to ensure ethical treatment of participants.
The FDA's Center for Drug Evaluation and Research oversees drug trials and requires all new therapies to undergo clinical testing before market approval. Other countries maintain their own regulatory frameworks, such as the Medicines and Healthcare Products Regulatory Agency in the U.K. or the National Medical Products Administration in China.
Clinical trial phases
Clinical trials typically proceed through a series of phases to assess different aspects of a treatment's safety and effectiveness:
Phase 0 (Exploratory or Early Phase I)
- Small group (fewer than 15 participants).
- Goal: micro-dosing to understand pharmacokinetics and preliminary human response.
- Not always required, but can streamline drug development.
Phase I
- From 15 to 30 participants (healthy volunteers or patients).
- Goal: Determine safe dosage range and identify side effects.
- Focus on safety rather than efficacy.
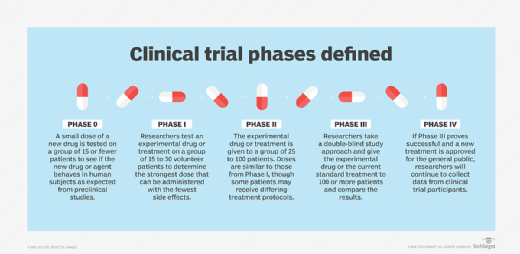
Phase II
- From 25 to 100 participants.
- Goal: evaluate treatment efficacy and continue safety monitoring.
- May include multiple treatment arms and protocols.
Phase III
- More than 100 participants across multiple sites.
- Goal: compare new treatment to current standard of care.
- Can include randomized, double-blind, placebo-controlled study designs.
- Results form the basis of regulatory approval.
Phase IV
- Conducted after a treatment is approved and commercially available.
- Goal: monitor long-term effects and gather additional safety and efficacy data in real-world populations.
On average, the full clinical trial process can take up to 10 years and cost hundreds of millions or even billions of dollars.
Clinical trial vs. clinical study
A clinical study is any research involving human participants with the goal of gaining medical knowledge. There are two primary types:
- Interventional studies (clinical trials). Researchers assign participants to receive specific interventions to measure outcomes.
- Observational studies. Researchers observe health outcomes in participants without assigning specific interventions.
For example, a clinical trial might assign one group to receive a new drug and another to receive a placebo. At the same time, an observational study might follow patients taking various treatments prescribed by their physicians.
Randomized controlled trials (RCTs)
A randomized controlled trial is the gold standard for evaluating treatment efficacy. In an RCT, the following conditions must be met:
- Participants are randomly assigned to intervention or control groups.
- Neither the patient nor the clinician (in double-blind studies) knows which group the patient is in.
- Results are compared to determine the effect of the treatment.
This approach helps eliminate selection bias and increases the reliability of results.
Eliminating bias in clinical trials
Researchers use several strategies to reduce bias and ensure reliable data:
- Randomization: Ensures comparable groups.
- Blinding: Keeps participants and/or researchers unaware of group assignments.
- Placebo control: Allows comparison against an inactive treatment.
- Standardized protocols: Ensures consistent treatment across sites.
These methods help protect the integrity of the trial and ensure that findings are based on objective results.
Who can participate in a clinical trial?
Clinical trials include eligibility criteria that define who can or cannot participate. Criteria may include a range of factors:
- Age, sex or specific health conditions
- Stage of disease
- Previous treatment history
- Laboratory test results
Participants must provide informed consent, which affirms that they understand the trial's purpose, procedures, potential risks and benefits before enrolling.
Participation is voluntary, and subjects may withdraw at any time.
Where to find clinical trials
Individuals interested in joining a clinical trial can search various websites:
- ClinicalTrials.gov. A database of federally and privately supported clinical trials in the U.S. and globally.
- ResearchMatch.org. An NIH-funded nonprofit that connects volunteers with approved studies.
- Cancer.gov. Contains a searchable database of clinical trials funded by the National Cancer Institute.
- Hospital websites. Many academic and regional medical centers list ongoing trials.
Patients should consult with their healthcare providers to discuss trial participation and suitability.
Learn about the five main types of clinical trial data-sharing platforms -- trial registries, results repositories, open data platforms, sponsor-specific and consortium-based platforms.