What is DICOM (Digital Imaging and Communications in Medicine)?
DICOM (Digital Imaging and Communications in Medicine) is an international standard protocol for the managing and transmitting medical images and related data. Many healthcare facilities use the DICOM electronic data interchange standard for a variety of imaging-reliant departments.
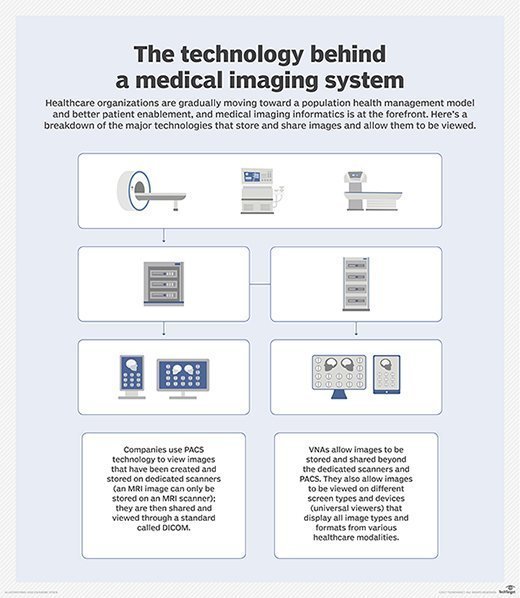
The DICOM standard makes it possible for different devices to share digital image information, regardless of device manufacturers or geographic location. Over the years, DICOM has facilitated the development of picture archiving and communication systems (PACS) that can securely store and digitally transmit electronic images produced by various hospital information systems, such as X-ray machines and CT scans. It has also contributed to the development of databases that contain diagnostic information and can be queried by a wide range of devices.
The DICOM standard
The DICOM standard is an evolving digital imaging management standard that facilitates the interoperability of medical imaging equipment and other systems, particularly equipment and systems used in the following fields:
- Radiology.
- Cardiology.
- Pathology.
- Dentistry.
- Ophthalmology.
- Related disciplines.
The diagnostic medical imaging systems used for image-based therapies, such as interventional radiology, radiotherapy and surgery, can also use DICOM to seamlessly exchange digital information.
DICOM enables device interoperability and seamless interchange of image information by providing a set of protocols for network communications as well as a set of media storage services for media communication. Devices that claim conformance to DICOM must follow these protocols and services. DICOM also provides the syntax and semantics of the various commands that can be exchanged using these protocols.
DICOM provides a multipart document that details the history, scope, goals and structure of the standard. This information is available on the DICOM website in multiple file formats (PDF, Hypertext Markup Language, Extensible Markup Language, etc.) and is revised and republished regularly less than the procedures of the DICOM Standards Committee.
As of this publishing, there are 22 parts to the current version of the DICOM structure covering everything from DICOM's overview, conformance, information object definitions, data structures and encoding, message exchange, media storage and file formats, data dictionary and content mapping resources. Some of the other important aspects included in DICOM PS3.1 2025c are information related to web services, service class specifications, application hosting, and imaging reports using the Health Level Seven International's Clinical Document Architecture (CDA).
What is DICOM used for?
DICOM is essential to communicate and manage medical images that are used for clinical analysis, diagnosis and to facilitate treatment as part of a patient's care plan. The information collected can be used to identify any anatomical and physiological abnormalities, chart the progress of treatment and provide clinicians with a database of normal patient scans for later reference.
DICOM's mission is to ensure the interoperability of systems used to produce, store, share, display, send, query, retrieve and print medical images, as well as to manage related workflows. Vendors that manufacture imaging equipment, such as MRI systems, imaging information systems and related equipment, often conform to DICOM. The standard can apply to any field of medicine where medical imaging technology is used.
The DICOM standard facilitates the operation and interoperability of devices in a networked environment. It can also accommodate new services and medical imaging applications. DICOM services can be transported using the DICOM Web Services application programming interface and HTTP Service. Additionally, DICOM metadata can be transported in real time using DICOM Real-Time Communication.
But not all medical images follow a DICOM format, which has led to the development of cross-document sharing, or XDS. An extension known as XDS-I is specific to imaging and allows the storage of multiple image formats. Many medical imaging system vendors offer features that interpret DICOM and non-DICOM formats.
Why DICOM is important
Today, DICOM is used worldwide to store, exchange and transmit medical images, enabling the integration of medical imaging devices from multiple manufacturers. Patient data and related images are exchanged and stored in a standardized format. Without a standard-based approach, it would be difficult to share data among different imaging devices because they would need to interpret multiple image formats, which can be time-consuming and may increase the potential for errors.
With DICOM, medical professionals have easier access to images and reports, allowing them to make better and more accurate diagnoses from anywhere in the world. In turn, patients can receive more efficient and better-quality care which can potentially improve patient outcomes.
Imaging information systems, in compliance with DICOM, have largely eliminated the need for film-based images and the physical storage required for film media. Instead, medical images, as well as related non-image data, can be securely stored digitally, whether on-premises or in the cloud.
Key features of DICOM
DICOM is a global standard for imaging interchange, meaning it can be used as is by any biomedical device. These devices might be in any geographic location and used in any medical specialty or local practice.
The standard includes mechanisms to handle different writing systems, character sets and languages. It can also handle devices that use different structures for addresses and people's names. It is possible to use the standard while also implementing any necessary localizations, such as specifying code sets for particular procedures to meet national or local health requirements.
DICOM allows many systems to exchange instances of persistent information objects, such as images. Even if the attributes of that instance are changed to make it easy for a particular organization to handle the item, its semantic content remains unchanged. Also, a globally unique object identifier identifies every instance of an information object. To create a new instance, the changes to the semantic content of an instance are defined. The new instance is then assigned a new object identifier, which is also globally unique.
DICOM history
DICOM was originally developed by the National Electrical Manufacturers Association (NEMA) and the American College of Radiology (ACR). In the 1970s, both NEMA and ACR recognized the need for a standard method for devices to seamlessly transfer and share images and associated information. This need was driven by certain technological developments that took place in the healthcare industry starting in the 1970s -- specifically, the introduction of computed tomography (CT) scanning and other digital diagnostic imaging modalities, and the increasing use of computers for clinical applications. At the time, different devices from different vendors produced digital images in a variety of digital image formats, making it hard for them to share images.
Once NEMA and ACR recognized the need for an imaging standard, they formed a joint committee in 1983 to develop the standard. The first version, ACR-NEMA standards Publication No. 300-1985, was published in 1985. It was revised in 1986 and again in 1988 to provide a consistent set of data formats for imaging data communication and interchange.
DICOM v2 (Publication No. 300-1988) was published in 1988. In addition to the hardware interfaces, software commands and data formats of v1, v2 also included command support for display devices and a hierarchy scheme to identify images. Subsequently, many other versions of DICOM were published
DICOM is a registered trademark of NEMA and is governed by the DICOM Standards Committee, an independent and international collaboration of users across all medical imaging specialties. The DICOM Standards Committee was formed in 1995 as a reorganized arm of the ACR-NEMA Joint Committee that originally proposed and developed DICOM in 1983.
As of this publication, the latest version of the standard is DICOM PS3.1 2025c.
DICOM and ISO
The DICOM standard is recognized by the International Organization for Standardization (ISO). Currently (July 2025), the second edition of the standard is in use and it is called ISO 12052: 2017 (Health informatics -- Digital imaging and communication in medicine (DICOM) including workflow and data management).
ISO 12052: 2017 will eventually be replaced by ISO/DIS 12052. As of July 2025, this new standard is in the enquiry phase with ISO members.
The updated standard will also reference two parts from DICOM version PS3.1 2025c. These are PS3.21 for transformations between DICOM and other representations and PS3.22 for real-time communication of DICOM content (DICOM-RTV).
Advanced imaging techniques are vital in healthcare, serving as a cornerstone for effective diagnostics and diligent disease monitoring. Explore healthcare imaging techniques, their uses and limitations.